
Dense Electron Catalysts
Specific conditions are required for the production of various dense electron and associated dense hydrogen states.
One method for producing dense electrons/dense hydrogen is to utilise a catalyst that can absorb the quantised energy released during an electron state transition, as proposed by R. Mills (mid 1980's).
The Mills catalyst model was based on elemental ionisation energies. A new method of identifying suitable catalysts was proposed by Hermanson (2017), based on augur electron energy data presented by Williams (2013), but predictions still remained inconsistent with low energy nuclear (LENR) and ultra-dense hydrogen (UDH) experimental observations.
Resolution of augur electron energy data to experimental observations and the LENR "small hydrogen" concept was achieved by Subtle Atomics based on Williams (2013), by a first principles extension of the Rydberg model to fractional n values (based on 13.6eV, not 27.2eV as proposed by Mills), and a modification to the Rydberg size states to an exponential rather than linear relationship (Brink, 2018).
The new dense hydrogen catalyst model is now consistent with experimental observations from super chemical, low energy nuclear (LENR) and low energy sub nuclear (LENSR) reaction systems.
Catalyst Tables
A catalyst database for the formation of dense electron/dense hydrogen states has been developed based on the above methodology.
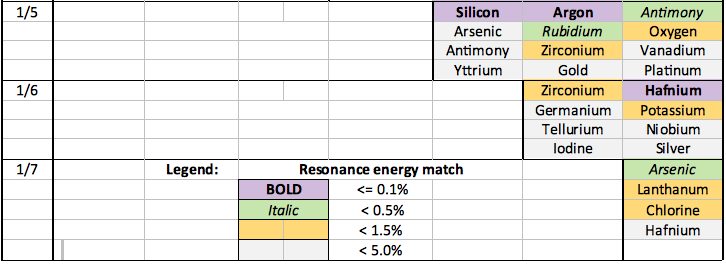
Selected examples of catalysts for transitions down to the primary electron state (n=1/8) are shown in the table below. Catalysts have been colour coded according to the percentage error in the energy match, refer to the legend below.
Close catalystic matches (i.e. <0.2%) are expected to generally be required to facilitate dense electron/dense hydrogen formation. These catalysts are shown as 'purple' in the table.

S. Brink 6th July 2018
Comprehensive Catalyst List
The Subtle Atomics catalyst model was explored by members of the LENR forum research in late 2018, and comprehensive catalyst list was developed using an automated Python code based method. This catalyst list is available for download via the link below.
Download
(Note that there are some slight variations in the tables' predictions as the table in the link above uses the Rydberg Energy of hydrogen, i.e. 13.605693eV, rather than the hydrogen ionisation energy)
References
Hermanson, E. 2017, pers. comm.
Williams, G. 2013, Electron Binding Energies for the Elements, Jefferson Lab, US Department of Energy
Available at: https://userweb.jlab.org/~gwyn/
Brink, S. 2018, Low Energy Nuclear Reaction Catalyst Identification Model, self published,
